THE SCIENCE OF THAUMATIN
OVERVIEW
Among sweeteners, natural sweet proteins are unique in that they are natural, often have high sweetness potency compared to sugar and decompose into a normal distribution of amino acids on hydrolysis. To date, thaumatin is the only commercially available ‘natural protein’ sweetener.
This versatile ingredient has a wide range of applications in foods and drinks and particularly in the field of taste modification and flavour enhancement.
STABILITY
Thaumatin is remarkably stable considering its proteinaceous nature. It is successfully applied in canning of pet food where it remains stable at temperatures of 120ºC and in coatings subjected to dry temperatures of 140ºC.
Thaumatin is stable under pasteurisation and UHT conditions. Further enhancement of its physical and chemical stability can be achieved through reformulation with protective compounds. Its stability under acid conditions, to lower than pH 2, is a useful feature - a result of its molecular structure.
PROPERTIES AND APPLICATION
Thaumatin’s properties can be usefully summarised under the headings of:
Enhancing
Masking
Synergism
Its applications are however less easy to categorise in the same way because its properties can act simultaneously and in many product applications are utilised together.
ENHANCEMENT
Thaumatin is effective in enhancing a wide range of flavour compounds, including savoury flavours and, in particular, “aggressive” flavours. Due to its ability to reduce the aggressive nature of these complex flavour compounds, thaumatin provides the opportunity to achieve higher levels of flavour in products without these being too aggressive and for instance, in coffee flavoured products, without generating bitter notes.
In savoury products enhancement is more complex. In addition to enhancement of flavours directly, thaumatin synergises with naturally present or added flavour enhancers such as 5’ nucleotides and monosodium glutamate (MSG). This adds a unique element of what the Japanese industry describes as “Umami” or, approximately translated into English, ”deliciousness”.
Thaumatin and Science
In addition to its use as a sweetener, thaumatin has become an important protein for basic science research. We describe below three aspects of thaumatin that are being explored: crystallisation, purity and taste.
A. Crystallisation
In 1994, it was discovered that adding the molecule l-tartrate to a solution of thaumatin causes the protein to crystallise rapidly. Bipyramidal crystals, shown in the photograph below, form in as little as twenty minutes. This is in contrast to most proteins, which takes days, weeks or months to crystallize—if they crystallize at all.
Caption: Bipyramidal crystals of Natex thaumatin grown with l-tartrate. The crystals are viewed under crossed polarisers with a lambda plate. The different colours are produced by the birefringence of the thaumatin crystals. The length of the white scale bar is 0.1 mm.
Since thaumatin crystallises so rapidly, scientists use it as a test case to study how protein crystals form and grow. Thaumatin crystals are also used to improve protein crystallography, which is the process of using x-ray diffraction from protein crystals to determine the three-dimensional structure of the protein. Thaumatin has seen it all—traditional, manual crystallisation methods and high-throughput robotic platforms; crystallisation experiments at high pressure and radiation damage tests at low temperatures. It has even been taken to space to find out whether weightlessness affects the quality of the crystals that are produced (see the figure below).
Caption: A crystal of thaumatin grown on the International Space Station under weightlessness (http://www.nasa.gov/mission_pages/station/research/experiments/APCFCrystal_Quality.html).
Thaumatin continues to be studied today in an effort to better understand protein crystallisation.
B. Purity
When carrying out research on proteins, it is important for the protein to be pure. This is especially true when trying to crystallise proteins. Unwanted contamination can lead to poorly formed crystals, aggregates (disordered clumps of protein) or in the extreme case, absolutely nothing because the impurities prevent the proteins from crystallising.
Unfortunately, a significant portion of the research on thaumatin, especially that related to protein crystallisation, has been done with impure protein. While the impure protein can still be crystallised, the quality of the crystals is poor, which makes it more difficult to use these crystals to determine the structure of the protein.
The thaumatin produced by Natex offers a way to overcome these difficulties. After a single purification step, it is possible to obtain large quantities of pure protein. For example, dynamic light scattering measurements (shown in the figure below) show that this purified protein is monomeric and free of any aggregates. It can be stored in solution at low pH for over a year without any degradation of the protein.
More information about the purity of Natex thaumatin can be
found in the article “Effects of Protein Purity and Precipitant
Stereochemistry on the Crystallization of Thaumatin” by N. Asherie,
C. Ginsberg, A. Greenbaum, S. Blass and S. Knafo, Crystal
Growth & Design, vol. 9, pp. 4200-4207 (2008), which is
available here: http://pubs.acs.org/doi/abs/10.1021/cg800616q
C. Structure and Taste
Thaumatin is exceptionally sweet. One molecule of thaumatin is about 100,000 sweeter than a molecule of table sugar (sucrose). Understanding why thaumatin is so sweet is an active area of research.
The detailed mechanism for sensing thaumatin’s sweetness is different than that of sucrose. The reason is that thaumatin and sucrose have different structures. Sucrose is a small molecule than binds to a site on the sweet receptor in the tongue similar to how a key fits inside a lock. Thaumatin, whose structure is shown in the figure below, is about five times larger (in radius) than sucrose and is too big to fit into the lock.
It might be thought that a small portion of thaumatin can mimic the key and fit into the lock. However, it has been shown that the sweetness of thaumatin does not come from a small, localized region of the molecule, but that numerous amino acids dispersed around the surface of the protein are responsible for its unusual sweetness. This suggests that thaumatin binds to a larger, external site of the sweet receptor; an intriguing hypothesis that is currently being explored.
STRUCTURE AND NATURE
Thaumatin, extracted unmodified from the Katemfe fruit using purely physical extraction methods consists of very closely related Thaumatin proteins. These have a single polypeptide chain or 207 “normal” amino acid residues linked with 8 disulphide bridges giving a molecular weight of around 22,000.
Thaumatin is completely digested by man and animal, which together with its “normal” amino acid sequence, accounts for its acceptance by regulatory authorities around the world as a safe, natural substance.
Thaumatin has the normal calorific value of a protein (4.1 Kcal/g) but in use is essentially non-calorific due to the very low levels required (parts per million). It is very basic with an iso-electric point of around 11.5 and freely soluble in water.
MASKING
Thaumatin’s masking effects, particularly of metallic or bitter tastes, are an important feature accounting for it’s widespread use in the human and animal food industry and with high intensity sweeteners and in particular with saccharin.
Thaumatin is particularly effective in masking the metallic aftertaste of saccharin, with this combination being used in a wide variety of products from tabletop sweeteners to animal feeds. In citrus fruit products, thaumatin has been shown to be very effective in masking the bitter elements of natural flavours from the fruit hence its application in products such as juices, yoghurts and deserts.
A good example of thaumatin’s simultaneous action is to be found in applications with vitamin C, often flavoured with citrus and sweetened with high intensity sweeteners. In this application thaumatin also masks the bitter notes and the sweetener aftertaste, enhances the flavour and also contributes to the sweetening. At high levels of inclusion but still in the parts per million ranges, thaumatin is effective in masking the taste of some pharmaceutical products in chewables and suspensions.
SYNERGISM
A clear synergism is shown when using thaumatin in conjunction with intensity sweeteners and with flavour enhancing compounds MSG and 5’ nucleotides. With aspartame for example, a commercial application showed sufficient synergy between thaumatin and aspartame that a thaumatin inclusion of 10 parts per million, the aspartame could be reduced by more than 30% to achieve the original sweetness level. In addition to the synergistic effects thaumatin improved the sweetness profiles of non nutritive sweeteners giving “body” to the sweeteners and masking the associated aftertaste as well as extending the sweetness and flavour profiles.
Thaumatin as the most potent form of sweetness available, and being the only natural high intensity sweetener available, does have some application as a sweetener in its own right, but due to a liquorice aftertaste being contributed at higher levels, its use as the only source of sweetness is limited to applications where the sweetness requirement is less than the equivalent of 10% of sucrose.
Above this level a taste contribution from thaumatin can become evident, which although desirable in some products, is not generally acceptable.
Applications in conjunction with natural low intensity, non-nutritive sweeteners are of interests in products requiring an “all natural” label. Thaumatin’s application in products in conjunction with added MSG and nucleotide compounds, or where nucleotides are naturally present, for instance in pet food, exhibit a high degree of synergy with these compounds
The additional masking effects of metallic flavours provide a range of applications in low sodium products where NaCl has been replaced in part with KCl.
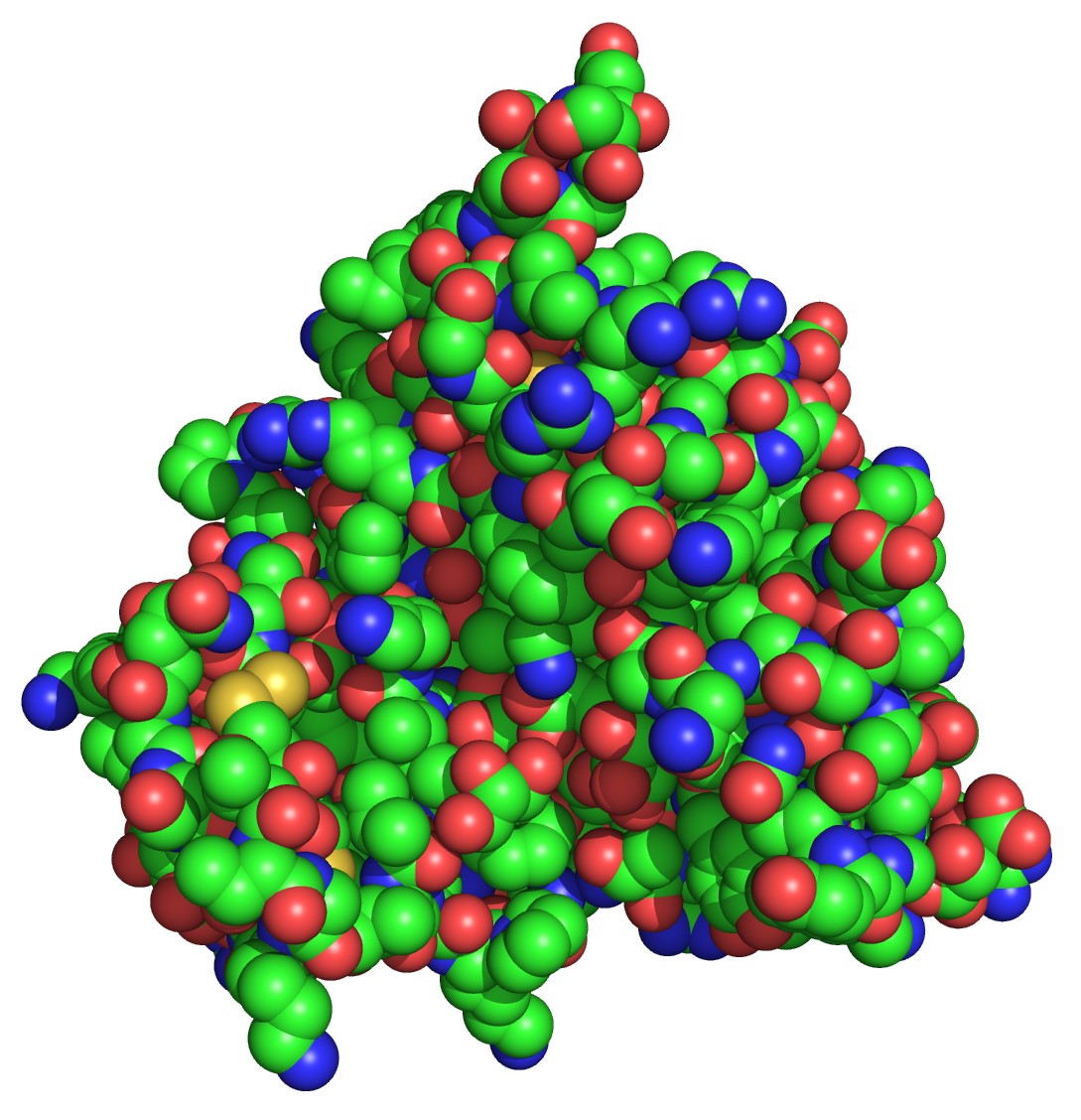
Caption: A space-filling model of thaumatin. The spheres are colour-coded by element: green (carbon), blue (nitrogen), red (oxygen) and yellow (sulphur). This structure was obtained using Natex thaumatin and is currently the highest-resolution structure available of the protein (0.94 angstroms). For more information see the Protein Data Bank: http://www.rcsb.org/pdb/explore/explore.do?pdbId=2vhk
TASTE MECHANISM
Thaumatin has a demonstrable effect on various groups of taste receptors, in various species of animal, for which it is used in feeding stuffs, as well as in man and is assumed to operate with the majority of taste receptors. This feature is a result of its particular structure. In the analogy, often used, of flavour molecules being “keys” and the taste receptors being “locks”, thaumatin appears to act as a “master key” in its action with all taste receptors.
Thaumatin’s effect in operating with all the taste receptors is the major reason for its wide range of applicability in situations requiring flavour modifications.
The electrical charge distribution on the thaumatin molecule is believed to be a major factor in the mechanism of interaction with both the taste receptor and the flavour molecules. This is demonstrated by the specific masking effects on the typically bitter unpleasant taste of metallic ions such as sodium, iron, potassium and its simultaneous enhancement of other free ion, e.g. chloride.
Its effect with more complex flavour compounds support the view that thaumatin’s mechanism operates according to the relative nature of the individual molecules present. This is particularly evident in its effect with flavours such as peppermint, ginger, cinnamon and coffee, which it enhances while simultaneously reducing the fiery, peppery or bitter elements associated with them.
Thaumatin’s high and rapid solubility in water and its tendency to increase salivation are additional physical features that increase the effect of flavours and aromas, particularly where physical contact with the taste receptors is limited.
The strength of the interaction between the thaumatin molecule and the taste receptors is believed to account not only for its potency effects in prolonging the effects of flavours and sweeteners.
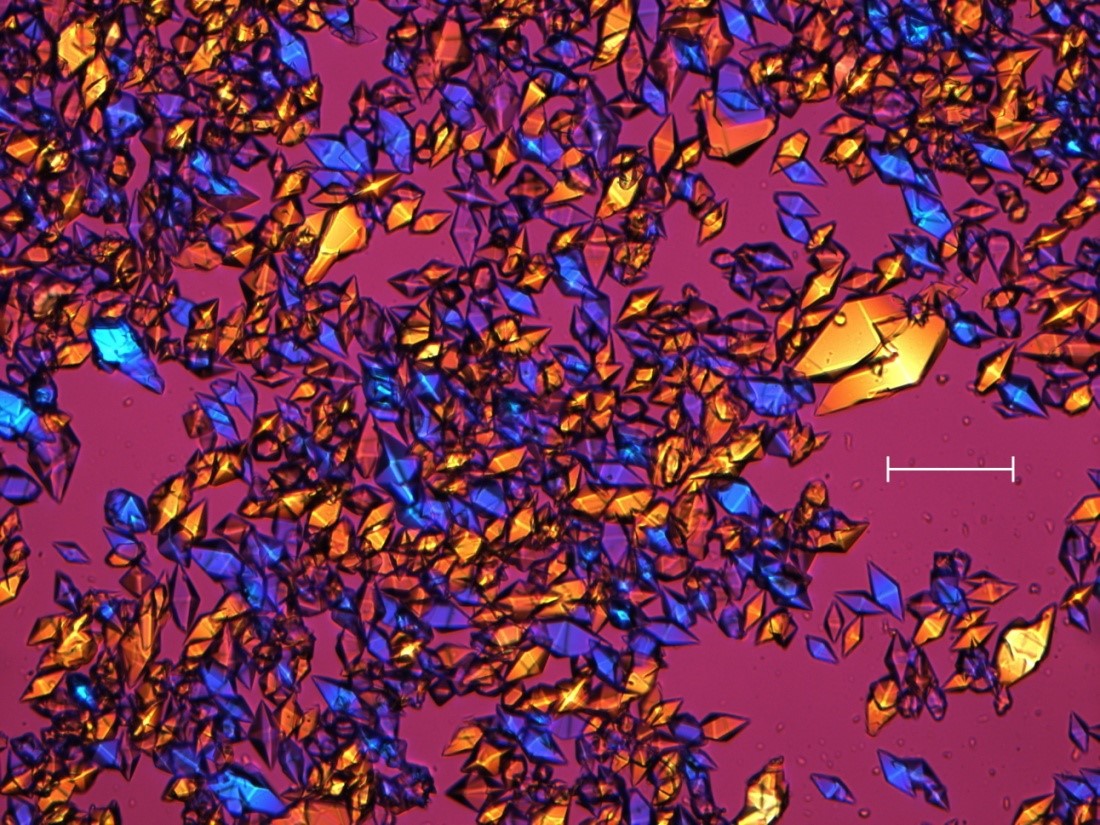
Caption: Bipyramidal crystals of Natex thaumatin grown with l-tartrate. The crystals are viewed under crossed polarisers with a lambda plate. The different colours are produced by the birefringence of the thaumatin crystals. The length of the white scale bar is 0.1 mm.

Caption: A crystal of thaumatin grown on the International Space Station under weightlessness .
www.nasa.gov/mission_pages/station/research/experiments/APCFCrystal_Quality.html
More information about the purity of Natex thaumatin can be found in the article “Effects of Protein Purity and Precipitant Stereochemistry on the Crystallization of Thaumatin” by N. Asherie, C. Ginsberg, A. Greenbaum, S. Blass and S. Knafo, Crystal Growth & Design, vol. 9, pp. 4200-4207 (2008), which is available here: http://pubs.acs.org/doi/abs/10.1021/cg800616q
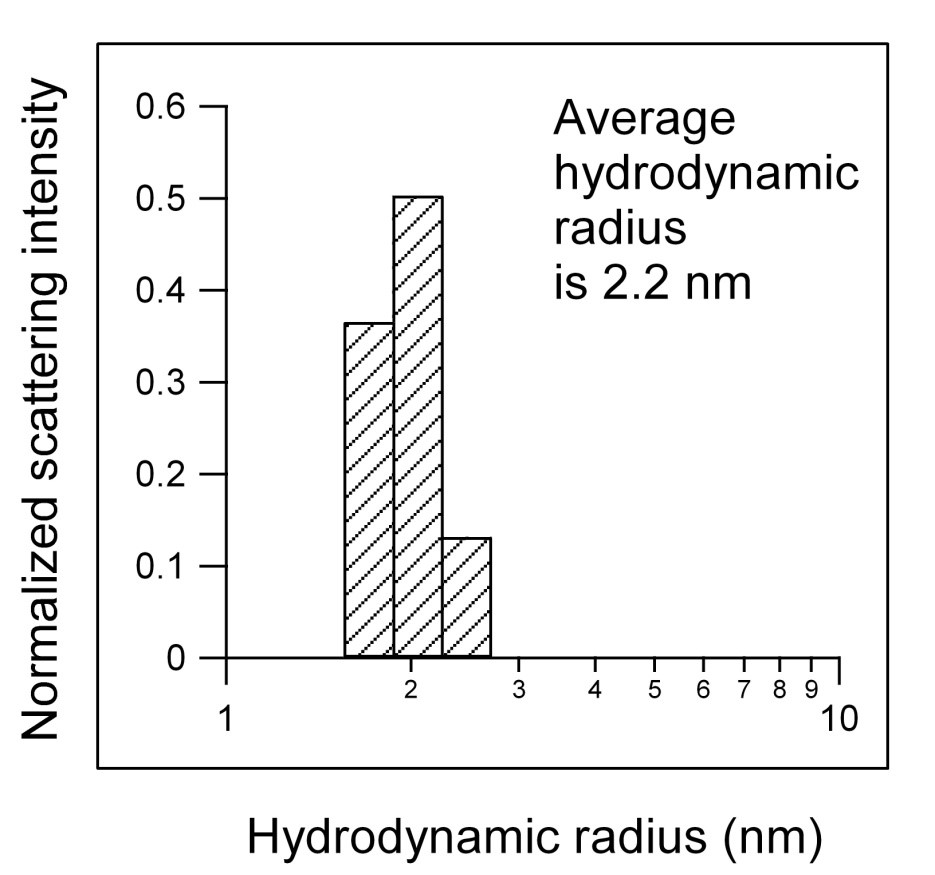
Caption: A histogram of the light scattering data for Natex thaumatin. The bars show the distribution of scattered light as a function of an effective size known as the hydrodynamic radius. The narrow distribution of sizes and the absence of any light scattering at larger sizes illustrate the high purity of the protein.